A Group on the Periodic Table Is Best Described as
The f-block columns between groups 2 and 3 are not numbered. Which of the following statements best describes the lements in Group 8A 18 of the periodic table.
Element Group.

. A group on the periodic table is best described as a Answers. The f-block columns between groups 3 and 4 are not numbered. Which sequence of elements is aranged in order of decreasing atomie radi.
The rows are called the periods while the columns are called the groups. The number of principal energy levels increases and the number of valence electrons increases. As elements in a group of the periodic table are considered in order from top to bottom the metallic character of each successive element generally.
There are two rows of elements found below the main body of the periodic table. A row of elements. Which phrase best describes a group or family in the periodic table.
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. Water is siphoned from a large tank and discharges into the atmosphere through a 50-mm diameter tube. The elements in the periodic table are divided into rows and columns.
Which statement best describes Group 2 elements as they are considered in order from top to bottom of the periodic table. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms ie. A group can be defined as a collection of elements with similar chemical or electronic properties.
Question 2 of 10 Which best describes the orthocenter of a triangle. The point where the three medians of the triangle. A student is given two unknown metals and asked to determine whether these two elements belong to the same group in the periodic table.
Measure only physical properties of the metals. They form ions of variable charge. Atoms of elements which are found in the same group typically have the same number of valence or outermost electrons.
Which of the following will help the student determine if the metals belong to the same group. The term is normally applied to a group of between six and nine elements boron silicon germanium arsenic antimony tellurium and possibly bismuth polonium astatine found near the center of the P-block or main block of the periodic table. The f-block columns between groups 3 and 4 are not numbered.
Gain electrons more readily and increase in nonmetallic character. Elements are listed in numerical order by atomic number. In chemistry a group also known as a family is a column of elements in the periodic table of the chemical elements.
Each group may be asked to collect the information about the elements of the group assigned to them. 1 Get Other questions on the subject. A The number of principal energy levels increses A Al Si P B 14 Na K C CI Br 1 D N C B the number of valence clectrons increases.
A The number of principal energy levels increases and the number of valence electrons increases. This means that a period is best described as a row of elements from the foregoing. Which statement best describes Group 2 elements as they are considered in order from top to bottom of the Periodic Table.
Each vertical column on the periodic table is called a group. There are 18 numbered groups in the periodic table. What is a group in periodic table.
Which statement best describes Group 2 elements as_ they are considered in order from top to bottorn of the Periodic Table. A elements that have the same melting point b elements that have the same number of protons in their nucleus c elements that are all in the same physical state at room temperature d elements that have the same number of electrons in their outermost orbits. In chemistry a group also known as a family is a column of elements in the periodic table of the chemical elements.
Physics 22062019 1130 ramirezzairap2u4lh. Contains elements that are among the most. Measure both chemical and physical properties of.
The phrase which best describes a period on the periodic table is. B The number of principal energy levels increases and the number of valence electrons remains the same. To answer this question we need to determine which of the statements provided best describes a group in the periodic table.
Its monatomic form H is the most abundant chemical substance in the Universe constituting. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. The number of principal energy levels increases and the number of valence electrons remains the same.
The end of the tube is b 21 m below the tank bottom which is a 74 m deep and. The y-intercept of gx is. Each horizontal row on the periodic table is called a period.
A group in periodic table is the column of element in periodic table. As the elements in Period 3 are considered from left to right they tend to. In chemistry a group also known as a family 1 is a column of elements in the periodic table of the chemical elements.
While working in a group of two the members are not getting along. Atoms contain a single valence electron. An example of an element group is the semimetals or metalloids which follow a zig-zag path down the periodic table.
There are 18 numbered groups in the periodic table. They exist as diatomic molecules. Good conductor of electricity.
Good conductor of thermal energy. The table represents the function fx. х 1 4 9 16 Oly fx -4 -3 -2 -1 0 If gx 45 8 which statement is true.
Although an element group often is defined as a column of the periodic table its common to refer to groups of elements that span multiple columns excluding some elements. There are 18 numbered groups in the periodic table. They exist naturally as single atoms.
Elements in a group of the Periodic Table are described below. A group number from the Modern Periodic table 1213-18. They are all liquids under normal conditions.
Which has a larger radius Na or Ne. Element groups defined this way do not always have the. The first ionization energy decreases down a column group and increases across a row period.
The Periodic Table Worksheet 1.

Color Coded Periodic Table Periodic Table Infoplease Com Periodic Table Periodic Table With Names Periodic Table Chart

Periods And Groups In The Periodic Table Chemistry For All The Fuse Periodic Table Chemistry Properties Of Matter

2006 Bent S Pln And Ple Front Step Periodic Tables Periodic Table Ordinal Numbers Chemistry

Periodic Table Interactive Activities In Google Slidestm For Distance Learning Distance Learning Interactive Activities Science Task Cards

Color Coding The Periodic Table Groups And Periods Periodic Table Color Coding Student Data

Dark Periodic Table Wallpaper Science Notes And Projects Periodic Table Periodic Table Of The Elements Science Notes

Color Coding The Periodic Table Groups And Periods Periodic Table Coding Electron Configuration

Periodic Properties Lab Determine Periodic Trends From Lab Data Chemistry Classroom Chemistry For Kids Chemistry

Periodic Table Printable Sample Periodic Table Printable Periodic Table Periodic Table Of The Elements

Color And Learn About The Periodic Table Layers Of Learning Physical Science Middle School Teaching Science Physical Science

Meet The Element Families Of The Periodic Table Alkaline Earth Metals Periodic Table Alkali Metal

Understanding The Periodic Table Through The Lens Of The Volatile Group I Metals Periodic Table Periodic Table Of The Elements Element Chemistry
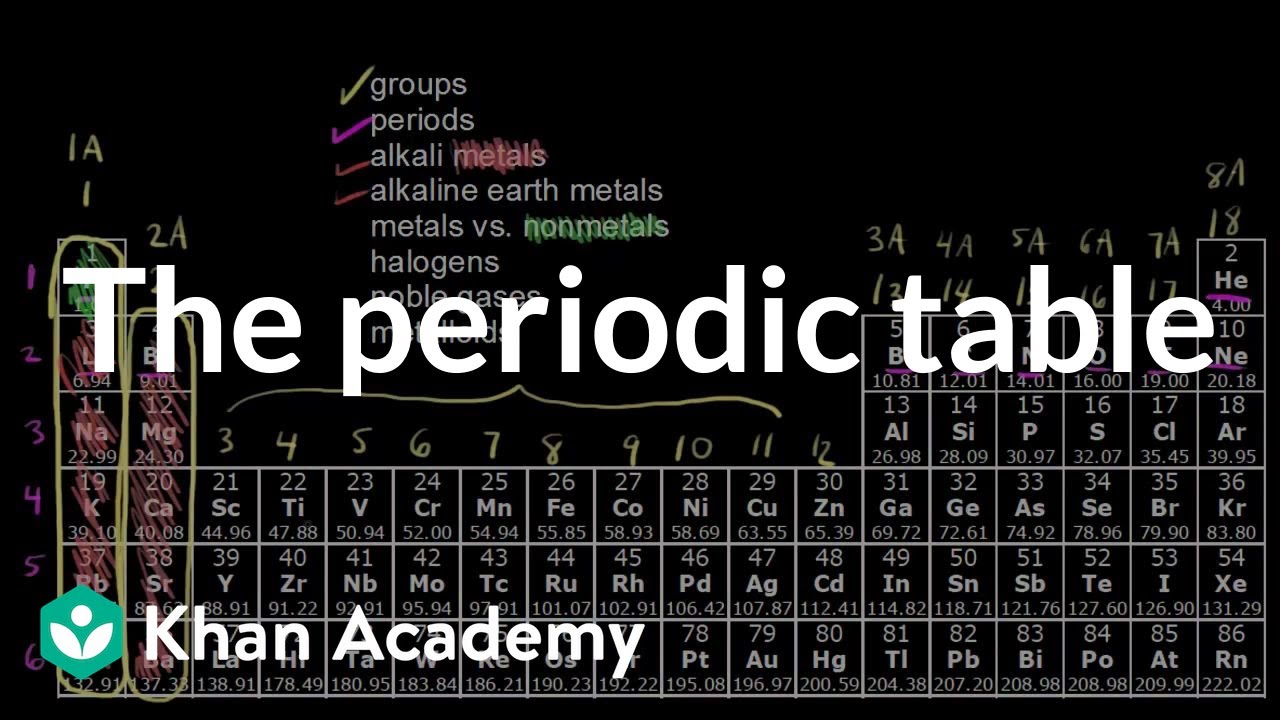
Definitions Of Groups Periods Alkali Metals Alkaline Earth Metals Halogens And Noble Gases How Metals Non Met Periodic Table Chemistry Element Chemistry

Organization Of The Periodic Table Worksheet Periodic Table Organization Worksheets

Worksheet Electron Dot Diagrams And Lewis Structures Answers Excelguider Com Electron Configuration Sequencing Worksheets Atom

Trends In Modern Periodic Table Class 10 Periodic Classification Of Elements Periodic Table Element Symbols Element Chemistry




Comments
Post a Comment